let's work together
Talent solutions for the global Life Sciences sector.
Our mission.
Changing lives by placing people at the heart of life sciences
Life sciences has never played a more important role for society, and with offices across the UK, Germany, USA and Australia we are committed to helping this vital sector solve challenging resource requirements and change more and more lives around the world.


Our Specialisms.
We work across the Pharmaceutical, Biotech and MedTech sectors, with a particular focus on the following specialist areas.
AI/ML & Data Science
We’re at the forefront of supporting the life sciences sector as it rapidly evolves through the integration of Artificial Intelligence and related emerging technologies. Our AI/ML recruitment specialism connects pioneering organisations, from techbio start-ups to global pharmaceutical leaders, with the world-class talent they need to drive innovation in drug discovery, clinical development, and precision medicine. Whether it's building out teams of AI/ML Scientists, Data Scientists, or placing AI/ML engineers into bioinformatics teams, we understand the unique demands of this high-growth, cross-disciplinary space.
Clinical
Clinical Operations is at the heart of what we do. We support some of the world’s largest CROs and Bio-Pharma companies sourcing Clinical Research Associates for international clinical trials across Phases I to IV.
Commercial
Our experienced Commercial Recruitment Teams support the leading CROs, Pharmaceutical and Biotechnology companies with sourcing sales and marketing specialists in Account Management, Fields Sales, Medical Sales, Market Access and Business Development.
Medical & Scientific
Our specialist teams support the leading Pharmaceutical, Biotech, and Contract Research Organisations with staffing in the Medical & Scientific space, in areas such as Medical Affairs, Medical Writing, Drug Safety and specialist scientists.
Quality Assurance & Tech Ops
Our specialist Quality Assurance and Technical Operations teams focus on a broad range of roles across Good Manufacturing Practice (GMP), Good Clinical Practice (GCP) and Good Laboratory Practice (GLP), as well as Supply Chain, Logistics and a range of Engineering roles.
Regulatory Affairs
Our specialist Regulatory Affairs teams support the leading Pharmaceutical, Biotech, and Contract Research Organisations with staffing in the Regulatory Affairs space, throughout the whole product life cycle from research to approval, through to commercialisation.
Commercial
Our experienced Commercial recruitment teams recruit for Medical Device companies across a range of disciplines including Marketing, Specialist Area Sales, Market Access and Business Development.

Quality Assurance / Regulatory Affairs
Our experienced recruitment teams recruit for Medical Device companies across a range of disciplines in the Quality Assurance and Regulatory Affairs space including Quality Engineers, Quality Managers, Regulatory Intelligence Specialists and Heads of Regulatory Affairs Specialists.
Research and Development
Our experienced recruitment teams recruit for Medical Device companies across a range of disciplines within the Research and Development space, including Project Managers, Validation Engineers and Principal Scientists.
Our Services.
Our independent, expert, and ethical approach to procuring talent has helped power the pioneering progress made by Pharmaceutical, Biotech and MedTech companies around the world. We focus on the following service areas.
Staffing Services.
-
Permanent Recruitment
Find out more -
Contract Recruitment
Find out more -
Executive Search
Find out more
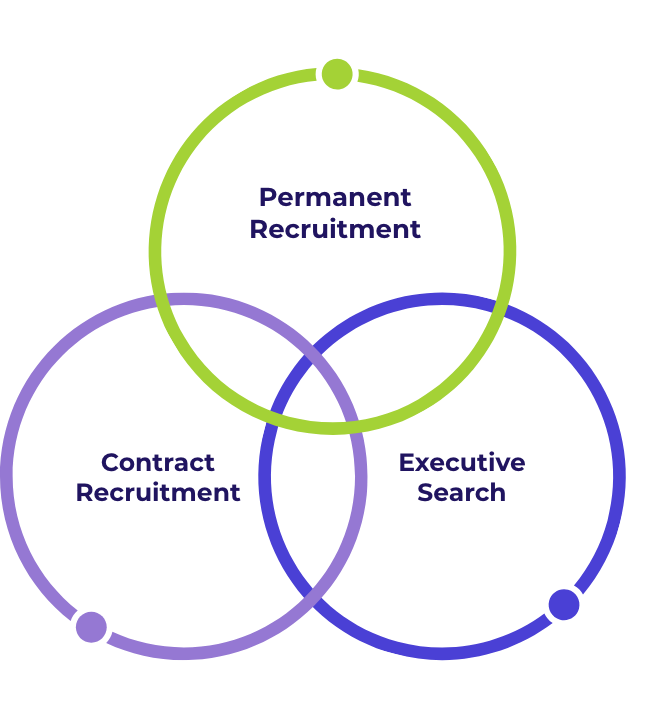
GxP Consulting Services
Our consulting services scope, staff, manage and deliver GxP solutions to our global client base.
We deliver across three distinct areas within life sciences:

Latest Jobs.
Read our latest insights
Awards & Accreditations.

i-Pharm provided us with both permanent and freelance candidates across all of our main service lines including Clinical Operations, Project Management, Biometrics, Regulatory Affairs and Drug Safety. They are one of the few recruitment agencies who can deliver for us globally.
Our Locations
With our HQ in London, and offices around the world, we have regional teams covering Europe, North America and the Asia-Pac region.
London, United Kingdom
contact@i-pharmconsulting.com+44 (0)20 3189 2299
Moor Place, 1 Fore Street Avenue,
London,
EC2Y 9DT
Sydney, Australia
contact@i-pharmconsulting.com+61 (0)2 8310 5840
Suite 30.01, Level 30, Governor Phillip Tower, 1 Farrer Place, Sydney NSW 2000, Australia
New York, USA
contact@i-pharmconsulting.com+1 212 461 4587
224 W 35th St, Ste 500 #3322, New York, NY, 10001











